
Boreskov Institute of Catalysis was founded in 1958 as a part of the Siberian Branch of the Russian Academy of Sciences. The founder and the first Director of the Institute till 1984 was academician Georgii Konstantinovich Boreskov.

One of the main activity areas of the Boreskov Institute of Catalysis is fundamental investigations in catalytic science to discover new principles of chemical reactions and to create innovative catalytic compositions and technologies.
Read more...

Boreskov Institute of Catalysis pays great attention to the training of young scientists. Each year more than 100 students and post-graduates are being trained at its research and educational facilities. The Institute collaborates with many educational organizations, including:
Read more...

For more than half a century, the Boreskov Institute of Catalysis is at a cutting edge of innovative R&D for chemical and petrochemical industries, energy power, environmental protection.
Read more...
8 December 2020
Researchers from Boreskov Institute of Catalysis develop catalysts based on the multi-wall carbon nanotubes for making hydrogen fuel from formic acid. The results of the study are published in International Journal of Hydrogen Energy.
Today molecular hydrogen is acknowledged as one of the most promising alternative sources of energy safe for the environment. The problem is that in its free state hydrogen is the lightest and one of the most low-boiling gases. It is explosion- and fire-hazardous, and this complicates its storage and transportation. The whole world is searching for the organic substances that would allow doing it safely. One of the most promising materials is formic acid. It provides high content of hydrogen (4.4 % wt, i.e. one gram of formic acid produces 0.044 g of hydrogen), chemical and thermodynamic stability, it is also non-toxic. In addition, formic acid is obtained by biomass processing, i.e. it is a cheap and renewable resource.
Decomposition of formic acid can go via two ways: dehydrogenation and dehydration. The first method is preferable, since during dehydration carbon monoxide is emitted, and the substance is poisonous for the catalysts of fuel elements. Dehydrogenation, in its turn, forms a mixture of gas products consisting of hydrogen and carbon dioxide.
“A crucial task now is the development of selective heterogeneous catalysts of dehydrogenation of formic acid. Today the catalysts based on noble metals such as silver, gold, palladium, platinum, ruthenium, are used. However, these compounds are expensive and hard to get, so the researchers today aim their studies at the searching for alternatives without expensive components. So, the catalysts based on transition metals and carbon materials suit the purpose perfectly,” — says Dr. Mariya Kazakova, Senior Researcher of Boreskov Institute of Catalysis.
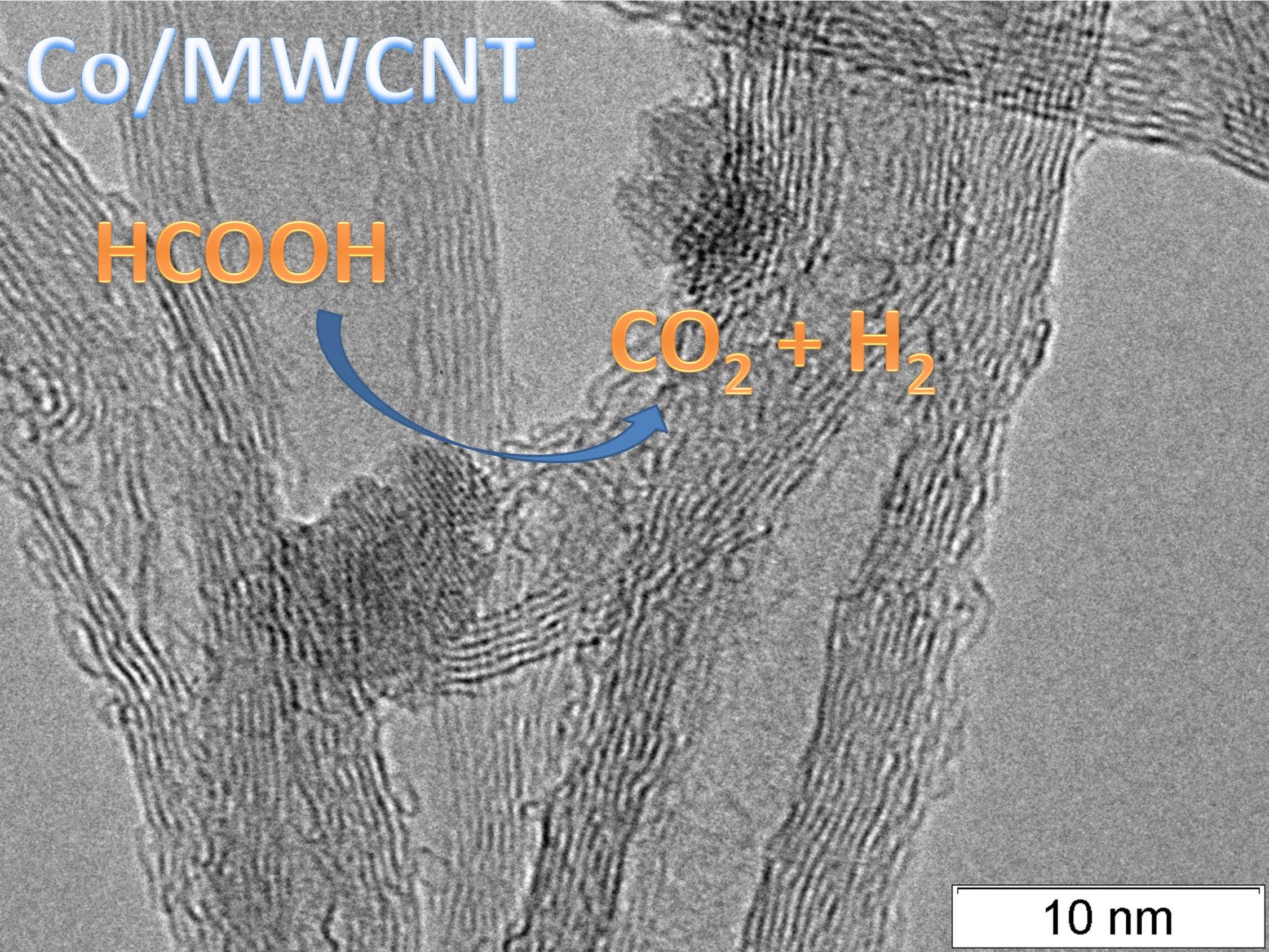
Microimage of cobalt-containing catalyst based on multi-wall carbon nanotubes that demonstrated the best activity in the reaction of dehydrogenation of formic acid
As the catalysts of dehydrogenation of formic acid the BIC researchers suggested the multi-wall carbon nanotubes (MWCNT) modified with the nanosized cobalt particles. The MWCNT differ from other carbon supports by several advantages. First, they have no acid-base centers that affect the formation of the active component and the course of reaction, i.e. can misdirect it. In addition, MWCNT have a large surface area and comprehensive porous structure (the latter can be represented by both inner channels and secondary porousness formed by the entwinement of separate nanotubes). The surface defects of the tubes can also provide additional spots for forming the metal particles. “Another advantage of the carbon nanotubes is that their surface and structure can be modified by inoculation of various heteroatoms and functional groups. Mainly, it is oxygen-, nitrogen-, sulfur-containing groups. This also promotes the favorable securing of the metal particles in the nanotube structure, which is crucially important for obtaining the active and selective catalysts of dehydrogenation,” — the researcher notes.
The MWCNT for the experiments are grown here, in Boreskov Institute of Catalysis. There is a plant in the Institute producing up to five kilograms of nanotubes a day, and there is everything necessary for improvement of their quality. In their work the scientists checked what influence is exerted by the structural characteristics (mean diameter, number of walls, imperfections) and functional composition of the carbon nanotubes over the securing of the cobalt nanoparticles and catalytic properties of the obtained samples in the reaction of dehydrogenation of formic acid.
“The best catalytic activity was demonstrated by the systems on the oxidized carbon nanotubes with the mean diameter of 9 nm. The catalytic activity was also increased by a growth of the cobalt content from 3 to 15 % wt., which is due to the formation of ~80% of Co particles on the outer surface of MWCNT with the mean diameter of 20 nm. Thus, the content of 15% of cobalt nanoparticles and their positioning immediately on the outer surface of the tubes is preferable,” — tells Mariya Kazakova.
Varying the MWCNT parameters the researchers hope to get the high-active and selective catalysts of dehydrogenation of formic acid without expensive components. “We consider searching for various systems based on non-noble metals and for possible synergetic effects from combination of several components in one composition. We would also like to study the influence of other functional processing of the carbon nanotubes over the securing of metal particles, the catalytic properties of the obtained systems. So far it is more a basic research, but it is quite possible that in several years it will acquire an applied character,” — says the researcher.
Source: "Nauka v Sibiri"