
Boreskov Institute of Catalysis was founded in 1958 as a part of the Siberian Branch of the Russian Academy of Sciences. The founder and the first Director of the Institute till 1984 was academician Georgii Konstantinovich Boreskov.

One of the main activity areas of the Boreskov Institute of Catalysis is fundamental investigations in catalytic science to discover new principles of chemical reactions and to create innovative catalytic compositions and technologies.
Read more...

Boreskov Institute of Catalysis pays great attention to the training of young scientists. Each year more than 100 students and post-graduates are being trained at its research and educational facilities. The Institute collaborates with many educational organizations, including:
Read more...

For more than half a century, the Boreskov Institute of Catalysis is at a cutting edge of innovative R&D for chemical and petrochemical industries, energy power, environmental protection.
Read more...
19 October 2021
With support from Russian Science Foundation the researchers of Boreskov Institute of Catalysis study how to use delamination of solid solutions in order to make efficient catalysts for oxidation of carbon monoxide and hydrocarbons. Such systems can become more active and cheap as compared with the traditional ecological catalysts based on noble metals.
The standard catalysts for oxidation of carbon monoxide and hydrocarbons used in car industry, thermal power plants and industrial enterprises are made on the basis of noble metals. But such compounds are expensive and do not always feature the necessary stability and resistance to the components of wastes.
Info: Solid solutions are a type of crystal solid substances where the atoms of various chemical elements are situated in one crystal lattice in strict order. These systems form a part of alloys which can have several kinds of solid solutions and single crystals of chemical compounds.
“We plan to develop catalysts based on transitional metals, in particular manganese-containing oxides. The idea of the study is to make a catalyst based on the laminated solid solutions – a system with strict order of atoms, where some elements can be replaced with others. We add manganese, cerium and oxygen into the oxide matrix. Then under various conditions through the stage of activation we try to pull manganese on the surface, as a result of which the nanoparticles of manganese oxide appear on the surface of the parent phase. We suppose that the systems based on the laminated solution will be higher activity as compared with traditional catalysts”, said Dr. Olga Bulavchenko, the head of the project, senior researcher of Boreskov Institute of Catalysis.
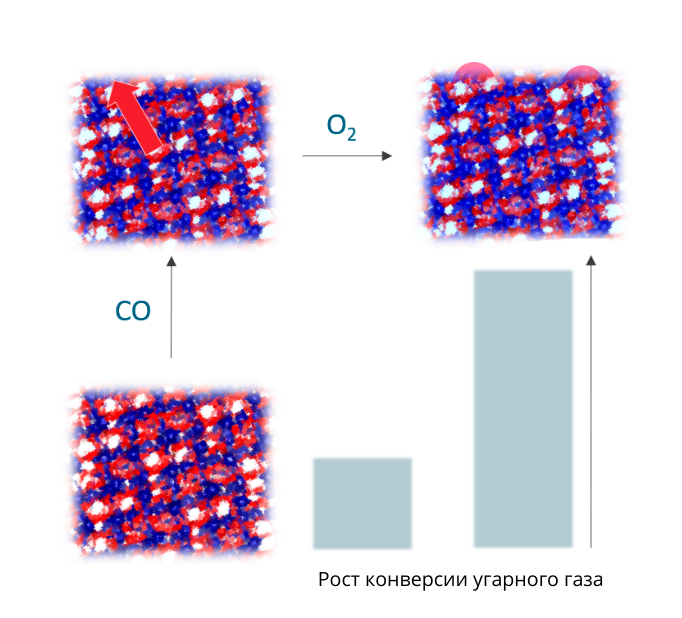
Scheme of lamination of solid solution and increase in CO conversion
The common application of the manganese nanoparticles on the matrix has its limitations.
“The manganese oxides sinter at temperature processing. If manganese comes out of the volume of solid solution, it fixes along with it – this way provides the small size of particles without sintering, i.e. the particles do not move freely on the surface and do not agglomerate”, explained the scientist.
According to Bulavchenko, synthesis and studying of the solid solutions is an important subject in the world scientific literature, but the researchers in Boreskov Institute of Catalysis decided to go by reverse. They studies the laminated solid solution and after cycles of reduction-oxidation noted that the catalytic activity of the compound increased.
The scientists will study the laminated solutions with the replaced cations with the help of diffraction method in the in situ mode that allows following the changes in the structure of catalysts immediately in the course of catalytic reaction and activation, in particular, watching the laminating of solid solution in real time.