
Boreskov Institute of Catalysis was founded in 1958 as a part of the Siberian Branch of the Russian Academy of Sciences. The founder and the first Director of the Institute till 1984 was academician Georgii Konstantinovich Boreskov.

One of the main activity areas of the Boreskov Institute of Catalysis is fundamental investigations in catalytic science to discover new principles of chemical reactions and to create innovative catalytic compositions and technologies.
Read more...

Boreskov Institute of Catalysis pays great attention to the training of young scientists. Each year more than 100 students and post-graduates are being trained at its research and educational facilities. The Institute collaborates with many educational organizations, including:
Read more...

For more than half a century, the Boreskov Institute of Catalysis is at a cutting edge of innovative R&D for chemical and petrochemical industries, energy power, environmental protection.
Read more...
9 December 2021
Scientists of Boreskov Institute of catalysis along with their colleagues from Ruhr University Bochum (Germany) proposed a strategy for enhancing the bifunctional ORR/OER performance of supported FeCoOx nanoparticles by tuning the physical and electronic properties of multi-walled carbon nanotubes (MWCNT). The oxygen reduction/oxygen evolution reactions are important for applications of alternative energetic — reversible fuel cells and rechargeable metal-air batteries, and in the long run this strategy can help to improve such devices. The study is published in top-rated journal ChemElectroChem (IF=4.590) and is its cover feature in 15/2021.
“The combination of nanostructured transitional metal oxides and carbon materials is a promising approach for synthesis of inexpensive, highly efficient, and stable bifunctional catalysts for the oxygen reduction/oxygen evolution reactions (ORR/OER). These reactions are used in the technologies of renewable energy sources — they occur in the reversible fuel cells and rechargeable metal-air batteries. Our approach will allow solving the problem of slow kinetics of ORR/OER that hinders the charging and recharging of the devices as well as reaching high efficiency and reversibility of reactions”, said Dr. Mariya Kazakova, senior researcher of BIC, one of the authors of the paper..
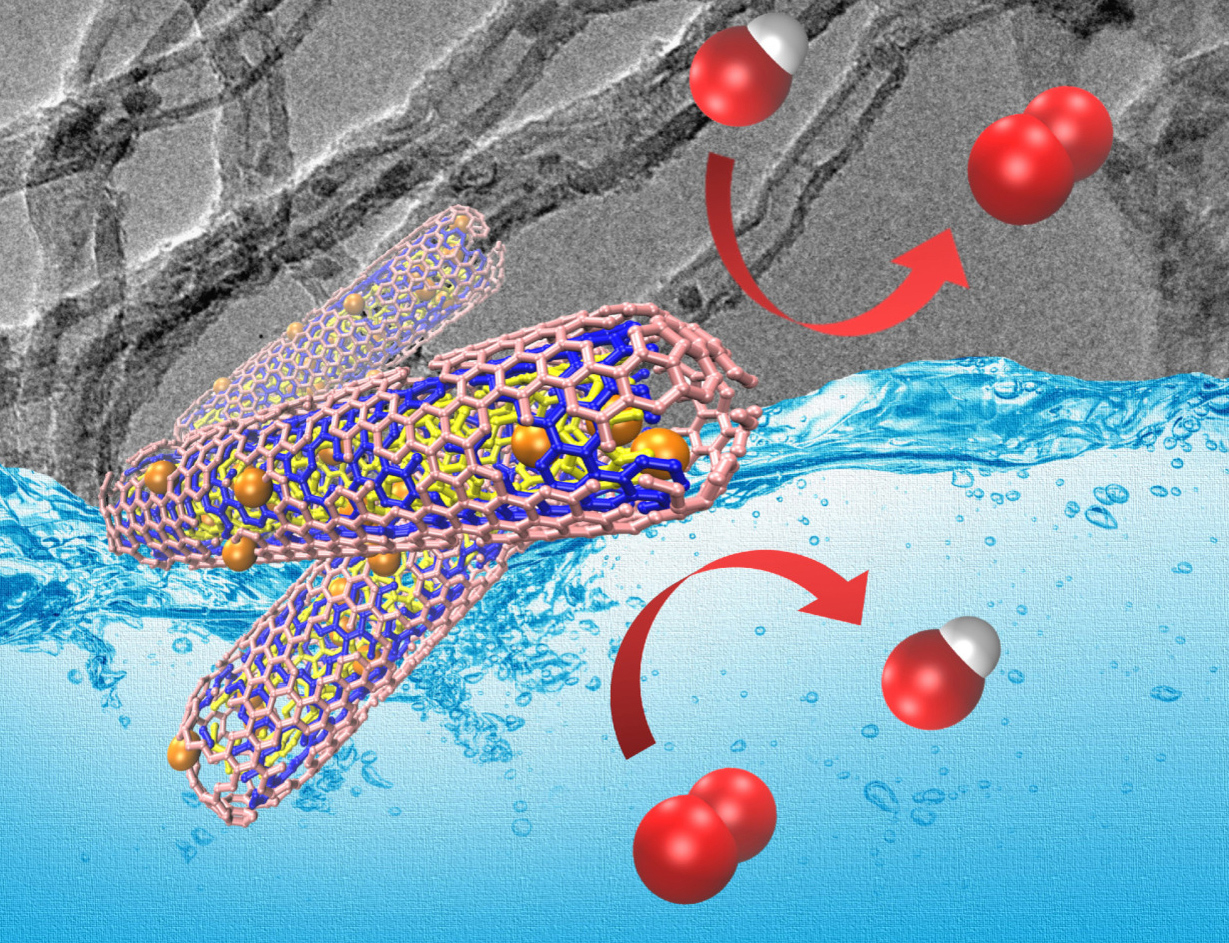
Bifunctional catalysts are the systems that catalyze various reactions of general chemical process. The scientists improved the parameter of bifunctional activity ORR/OER by developing catalysts based on nanoparticles of mixed oxide of iron and cobalt (FeCoOx) supported on multi-walled carbon nanotubes (MWCNT). The basic strategy was in the adjustment of MWCNT properties by nitrogen doping during their synthesis in the presence of ammonia and subsequent oxidative functionalization.
“The results showed that the oxidative processing helped to provide a precise control over dispersion and localization of the nanoparticles in the structure of nanotubes. In its turn, the optimal degree of nitrogen doping resulted in increased bifunctional activity due to enhanced electrical conductivity as well as improved catalyst stability in both OER and ORR conditions”, explained Mariya Kazakova.
According to Mariya Kazakova, varying the ammonia concentration allowed controlling the structure, surface chemical composition, and defectiveness of nanotubes. The oxidative processing allowed additional adjustment of texture characteristics and defectiveness of MWCNT as well as provided the hydrophilic properties of their surface which increased the dispersion of FeCoOx nanoparticles.