
Boreskov Institute of Catalysis was founded in 1958 as a part of the Siberian Branch of the Russian Academy of Sciences. The founder and the first Director of the Institute till 1984 was academician Georgii Konstantinovich Boreskov.

One of the main activity areas of the Boreskov Institute of Catalysis is fundamental investigations in catalytic science to discover new principles of chemical reactions and to create innovative catalytic compositions and technologies.
Read more...

Boreskov Institute of Catalysis pays great attention to the training of young scientists. Each year more than 100 students and post-graduates are being trained at its research and educational facilities. The Institute collaborates with many educational organizations, including:
Read more...

For more than half a century, the Boreskov Institute of Catalysis is at a cutting edge of innovative R&D for chemical and petrochemical industries, energy power, environmental protection.
Read more...
20 June 2022
The scientists of Boreskov Institute of Catalysis supported by the Russian Science Foundation study the reaction ability of multicomponent alloys in synthesis of carbon nanomaterials for design of catalysts of new type. The results of the study are supposed to be used for solving the problems of ecology, industry, and hydrogen energetics.
Multicomponent alloys, or high entropy alloys (MCA), are the systems containing 4-5 and more metals in similar concentrations. They can feature unique physical characteristics. Lately such systems are of great interest for scientists in terms of perspective to use in a number of catalytic reactions and electrocatalytic applications.
The academic novelty of the project is in that earlier such alloys were not used for synthesis of carbon nanomaterials. “Interesting that these alloys contain nickel, cobalt, iron, those very metals that are well known as catalysts for making carbon nanotubes, nanofibers, etc. But to this moment they were not used for synthesis of such materials. We are going to synthesize such alloy systems containing four, five, and more components for studying their ability to transform into active catalysts of synthesis of carbon nanostructures, first of all, carbon nanofibers”, says Dr. Ilya Mishakov, the head of the project, leading researcher of BIC.

How to prepare MCA
According to Ilya Mishakov, to realize various approaches to synthesis of the target alloys the project cooperate the employees of the Institute of Inorganic Chemistry (Novosibirsk) and Institute of Strength Physics and Materials Science (Tomsk) having the necessary competences. The project participants will use three ways of making MCA – thermolysis of multicomponent precursors, joint exploding wire method, and mechanochemical metal fusion.
Thermolysis of multicomponent precursors is made in the Institute of Inorganic Chemistry. The core of the method is that chemical reagents containing the necessary metal components are mixed, annealed, reduced, and yield the alloy.
MCA will also be produced in Tomsk with the joint exploding wire method from various metals. “In this process we take wires from necessary metals/alloys, intertwist them and disperse them by flashing high density current. As a result of the joint dispersion of the wires of various metals/alloys the particles of multicomponent alloy appear”, tells Ilya Mishakov.
The third method is the mechanochemical metal powder fusion. It engages planetary mill with great acceleration of milling bodies, which promotes the efficient fusion of components with each other.
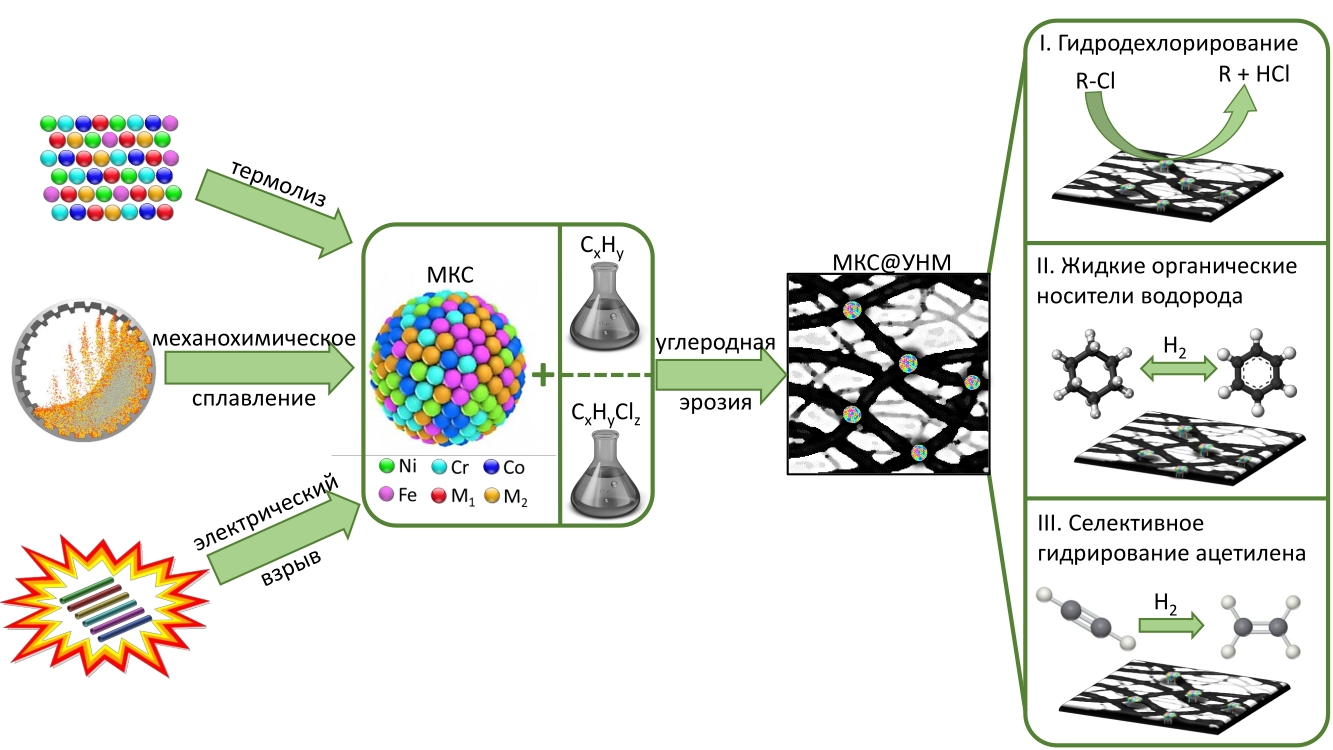
Area for using new catalysts
The main purpose of the project is to study the reaction ability of MCA in synthesis of carbon nanomaterials for making new type of supported catalysts. When the alloy is thermally processed in carbon-containing atmosphere, it disperses, and this leads to formation of active particles with catalytic properties. Carbon fibers grow on the active alloy particles. As a result of the process a composite material forms consisting of carbon nanofibers and MCA. Such systems can be further used as catalysts with a wide range of usage.
The scientists study how the new catalysts can be used for deactivation of chlorine-aromatic compounds.
“These systems can be used for adsorption and hydrodechlorination of chlorine-aromatic compounds. We will test the MCA supported on carbon in the processes of deactivation of chlorine-substituted hydrocarbons that can spoil water and make it unusable and unsafe for biological objects even if their quantity is small when they get into water bodies after technological accidents”, explains Mishakov.
The second direction is related to the process of selective hydration with ethylene production from acetylene, says the scientist: “In this process a triple bond is selectively hydrated, and ethylene is produced. Preparation of the raw material by selective hydration is one of important processes in industry”. This part of the work will be done in Center of New Chemical Technologies of BIC in Omsk.
Finally, the catalytic systems based on MCA can be promising for hydrogen storage. “Hydrogen storage is a rather complicated problem. One of the ways is based on the use of the so-called liquid organic hydrogen carriers that are able to reversibly take and give hydrogen. These processes also require catalysts, and the MCA-based systems can be promising”, tells Ilya Mishakov.
The processes of hydrodechlorination and hydration often use catalysts based on noble platinum metals. It is expected that the new systems will present a more available alternative. “The noble metals like palladium can be replaced with a set of very cheap ones – nickel, iron, chrome, etc., in various combinations. We search for combinations of metals that would work better ensemble providing the same activity as noble metals, or even higher”, notes the head of the project.