Spectral Methods
Homogeneous and heterogeneous catalysts, reactions with their participation.
Bruker AVANCE-400 (magnetic field 9.4 T) and Bruker AVANCE-400/microtomograph (magnetic field 9.4 T) NMR spectrometers for liquid-state and solid-state spectroscopy with a representative set of probeheads for any applications:
1. Home-built high-temperature (up to 600°C) probeheads for studies of molten state.
2. High-speed probeheads MAS: 7 mm - for rotation frequency up
3. MAS experiments under controlled atmosphere conditions in sealed ampoules.
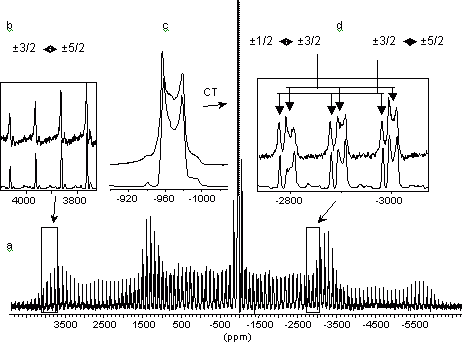
4. Solid – state NMR technique for quadrupolar nuclei with a half-integral spin:
Sample volume – from 0.1 to 5 cm3 depending on nucleus type.
Prof. E.P. Talsi. In situ NMR and EPR study of the structure and reactivity of the active intermediates in homogenous catalytic oxidation of alkanes and alkylbenzenes, olefines epoxidation and polimerization reactions.

Prof. M.A. Fedotov. NMR study of the coordinate compounds structure for molybdenum, tungsten and metals of platinum group in the solutions and their catalytic properties. The author of the monographs “Nuclear magnetic resonance in solutions of inorganic compounds” and “Nuclear magnetic resonance in inorganic chemistry”.
Prof. O.B. Lapina. Solid-state and high-temperature NMR studies of the structure and mechanism of formation of the active sites of heterogeneous catalysts.
Prof. A.G. Stepanov. Study of hydrocarbon conversion on solid catalysts, reactions mechanism and products analysis including in situ.
Dr. R.I. Maximovskaya. Structure and conversions of polyoxometallates in solutions according to the data of polynuclear NMR spectroscopy.
Dr. K.P. Bryliakov. Study of structure of intermediates in reactions of asymmetric oxidation.
Dr. D.A. Babushkin. NMR spectroscopy researches of intermediates in homogeneous catalytic reactions of oxidation and polymerization, determination of structure for organic, metalorganic and complex compounds, the analysis of functionalized polymers.
Original technique for estimation of the properties and the active sites concentration on different oxide catalysts surface using non-traditional spin probes.
Volume of the liquid or solid sample over 1 cm3.
Prof. E.P. Talsi. In situ NMR and EPR study of the structure and reactivity of the active intermediates in homogenous catalytic oxidation of alkanes and alkylbenzenes, olefines epoxidation and polimerization reactions.
Prof. A.M. Volodin. EPR study of radical and ion-radical reactions on oxide catalysts surface (dispersed oxides); development of spin-probes application techniques for heterogeneous catalysts active sites investigations.
Dr. K.P. Bryliakov. EPR studies of kinetics and mechanism of the reactions catalyzed by transition metal complexes. EPR of transition ions with S > 1/2.
Dispersed ferro- and ferrimagnetics, magnetic layers, any solid and liquid samples, containing magnetic particles.
ESR spectrometer Bruker-200, ESR spectrometer Bruker-EMX (from the EPR Center of Collective Use in Siberian Brunch) allow to study in-situ at temperatures from 77 K to 500 K.
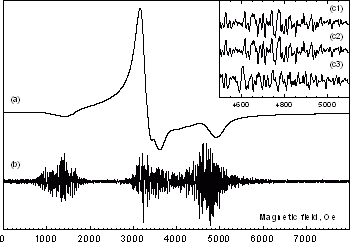
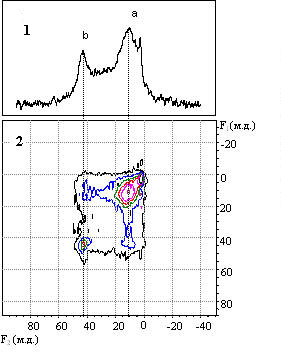
The FMR spectrum of Ln0.7Pb0.3MnO3 single crystal:
(a) – general view, (b) – the FMR fine structure obtained after the subtraction of the smooth background component.
In the insert (c1), (c2) – a sequence of FMR FS spectra obtained in independent registration series at unchangeable specimen position in the spectrometer cavity, (c3) the specimen is turned by 5°, Treg=296 K.
Maximal size of solid sample is 8 mm.
Prof. V.F. Yudanov and Dr. O.N. Martyanov. Dispersed magnetics, magnetic layers.
Solutions and solids, inorganic and organic substances.
Carl Zeiss Jena UV-VIS Specord M40 Spectrophotometer with a diffusion reflection accessory allows to record ultra-violet and visible (UV-VIS) spectra of solid samples in the range 45000 – 11000 cm–1.
Shimadzu UV-VIS 2501 PC Spectrophotometer with a diffusion reflection accessory, operation range – 53000 – 11000 cm–1.
Spectrolab Interspec 2010 NIR Fourier spectrometer with a diffusion reflection accessory allows to record the solid sample spectra in the near infrared region (NIR) 10000 - 3500 cm–1.
For UV-VIS: powder – not less than 1 cm3; non-transparent film – 2x2 cm2.
For near IRS: powder – not less than 0.5 cm3; film – 1x1 cm2.
For UV-VIS, NIR: solution – about 1 cm3, concentration of the substance to be defined in the solution – 10–5 mol/l and higher.
Prof. E.A. Paukshtis and T V. Larina. Electron and coordination state of the cations and nature of its bonding with ligands in oxide heterogeneous catalysts and solutions.
Dr. G.V. Odegova. Stabilization of the surface complexes of transition metals.
Varian FTIR-800 and Bruker Vector-22 IR Fourier-spectrometer are adapted for the study of solid surface. The operating range is 400–7800 cm–1, resolution – up to 1 cm–1, time of 1 spectrum recording is 1 second.
Shimadzu 8300 IR Fourier spectrometer is used for these purposes as well. The operating range is 300–6000 cm–1. Resolution – 1 cm–1, signal/noise ratio is higher than 2000. Time of 1 spectrum recording is 3 seconds. The surface is studied by the methods of in situ transmission (in the temperature range from –196 to +700 oC) and diffusion reflection (from –60 to +200 oC) in the controlled gas mixtures or vacuum.
The determination of gas molecule concentration to 1 ppm in a small pan
Original methods have been developed for the quantitative measurement of surface acid properties by low-temperature CO adsorption. (E.A.Paukshtis “Infrared spectroscopy in the heterogeneous acid-base catalysis”, Novosibirsk: Nauka, 1992)
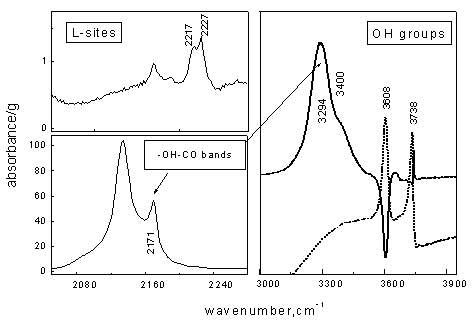
An example of IR spectra observed in the course of CO adsorption over H-ZSM-5 zeolite. IR spectra of the initial OH groups are shown by the dotted line. The solid line corresponds to the spectra of OH groups in H-complexes with CO.
From the spectra it is evident that the zeolite is characterised by two types of Lewis acid centers (bands 2217 and 2227 cm–1) and two types of Brönsted acid centers (OH group bands 3294 and 3400 cm–1). Such a subtle difference between acid centers is impossible to register by other methods.
Weight of the solid sample – 500 mg, for gases – 100 cm3.
The spectra of gaseous, liquid and solid samples in the frequency range 230 – 4000 cm–1 are acquired by Bomem IR Fourier-spectrometer. The program for spectra analysis allows to acquire high quality spectra for very dilute organic solutions. With CCl4 as a solvent it is possible to study the solutions with the concentration up to 0.5 × 10–3 mol/l.
Weight of solid sample (without considering inert support) – not less than 3 mg.
Volume of solution – not less than 2 ml and solvent used – not less than 2 ml.
For photoacoustic IR spectra acquisition the volume of powdery sample is 1 cm 3.

Prof. E.A. Paukshtis. Quantitative basis of the definition of surface acid-base properties, all fields of IR spectroscopy of adsorbed molecules. The author of monograph “Infrared spectroscopy in heterogeneous acid-base catalysis” (in Russian).
Prof. E.S. Stoyanov. Composition and structure of molecular associates and complexes in water and organic solutions, and on the interface of solid-gas, solid-liquid and liquid-liquid.
Dr. E.B. Burgina. Theoretical analysis of experimental vibrational spectra, experimental IR (including NIR) and Raman spectral investigations.
Dr. G.N. Kustova. Analysis of defective oxides structure with IR spectroscopy.
Dr. D.V. Kozlov. Study of the chemical processes on oxides surface (TiO2).
RF-5000 Spectrofluoremeter (Shimadzu, Japan). The wavelength range of the excitation monochromator is 200–700 nm. The wavelength range of fluorescence monochromator is 200-700 nm. The xenon lamp with a capacity of 150 wt serves as an emitter. Recording of the spectra of emission, excitation, synchronous spectra is accomplished.
Prof. D.I. Kochubey.
Dr. G.V. Odegova.