Thermal Gravimetry (TG) and Differential Thermal Analysis (DTA)
Objects of study
Various liquid and solid samples.
Applications
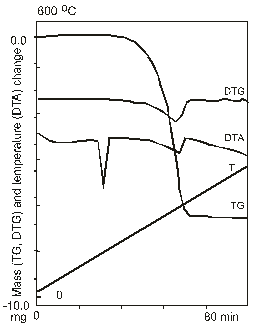
During the continuous programmed heating are defined weight variation (TG) and effects of heat adsorption and evolution (DTA) as a result of various physical and chemical sample transformations.
Instrument facilities
STA-449 (Netzsch, Germany), Q-1500 and C (Hungary) derivatographs with the following characteristics:
- Sample heating from the room temperature to 1000–1500 °C.
- Temperature increment rate from 0.6o/min to 20°min for Q-1500 and C and to 50°min for STA-449.
- Weight variation is estimated with an accuracy of up to ± 0.1 mg for Q-1500 and C, 0.1% for STA-449; the limits of weight estimation (balance sensitivity) may vary from 20 to 2000 mg.
- Measurement is carried out in the air atmosphere or inert or reductive gas flow.
Weight of the sample (TG), differential temperature (DTA) and weight derivatives are simultaneously and permanently recorded.
Samples requirements
Sample in the form of powder, granules, liquid in the quantity from 5 to 1000 mg.
Leading scientists and their research interests
G.S. Litvak. Hydroxide, oxide and carbon containing systems.
Temperature-Programmed Methods: Reduction (TPR), Oxidation (TPO), Desorption (TPD)
Objects of study
Oxide and metal catalysts both bulk and supported.
Applications
The determination of number and quantity of surface and volume forms of active component differed by the valent state, coordinational environment and dispersity.
Instrument facilities
Standard b>TPR method includes treating of the sample in the flow of oxygen or inert followed by temperature programmed reduction in the hydrogen-argon or CO/He flow with the measurement of hydrogen or CO consumption. The rate of heating from the room temperature to 1200 K may vary from 2 to 20 degrees/min.
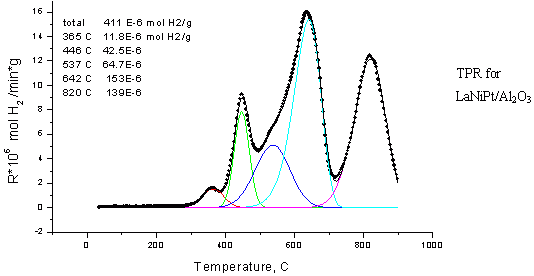
The following related variants of TPR method are available:
- Temperature programmed oxidation (TPO) – for the reduced samples .
- Temperature programmed desorption (TPD) – for the study of chemisorption of reagents and reaction products.
Samples requirements
The weight of the sample from 10 to 500 mg depending on the reducible component content.
Leading scientists and their research interests
Dr. V.A. Rogov. Oxide and metal catalysts.
A Set of Calorimetric Methods for the Study of Dispersed Systems and Reaction Heats
Applications
- Measurement of the specific heat of solid and liquid substances.
- Direct heats measurement: catalytic reactions and their stages; gas and vapour adsorption; wetting and dissolution of porous and dispersed solids; liquids mixing; combustion in oxygen of organic substances (individual compounds and their mixtures), natural and synthetic fuels and others.
- Combination of calorimeters with the vacuum units for the measurement of thermal effects at low pressures as well as flow-pulse micro catalytic units with simultaneous chromatographic analysis of reagents and catalytic reaction products.
Instrument facilities
- Calvet high temperature calorimeter (“Setaram” France): temperature range 40–500° C, in power 10–100 μW.
- Calvet calorimeter DAC-1A (Chernogolovka, Russia) and calorimeter MID-200 (“Etalon” plant, Kazakhstan): temperature range 20–200° C, in power 1–10 ?W.
- Combustion calorimeter with a bomb (“Etalon” plant, Kazakhstan).
Samples requirements
For thermal effects measurement the sample weight is to provide the total sample area not less than 5–10 m2. For specific heat estimation the sample weight – not less than 100 mg.
Leading scientists and their research interests
Prof. Yu.I. Aristov. Thermochemistry adsorption and catalytic reactions on the surface of metal and oxide catalysts.
Differential Scanning Calorimetry
Objects of study
Liquid and solid samples in the form of powder, crystals, plates et al.
Applications
- Measurement of the specific heat.
- Measurement of melting/crystallization temperature and heat.
- Phase diagrams determination.
- Determination of crystallinity extent of solid samples.
- Kinetic study, the measurement of thermal effects in topochemical reactions.
- Definition of the samples purity by melting/crystallization temperature and enthalpy.
Instrument facilities
Differential scanning calorimeter DSC 404 C Pegasus (NETZCH Gerätebau GmbH). Temperature range: –120 ... 700 ºC; RT ... 1500 ?C. Heating rate: 0.1–50 K/min. Atmosphere in work chamber: dynamic, oxidative, reducing, inert, vacuum. Precision: < 2.5% for enthalpy changing, ± 3 K for temperature. Sensitivity: 1–10 mcW. Base line linearity < ± 2.5 mW.
Registration forms for results obtained:
- time dependence of the rate of heat absorbtion/desorbtion in the sample (mW/g);the data are plotted in real time;
- simultaneous record of data in the file that allows to treat it with using software Proteus.
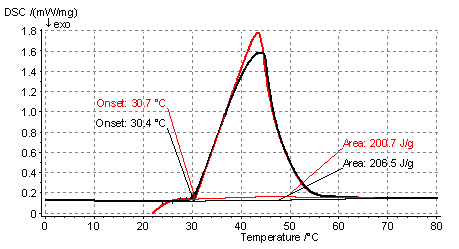
Endothermic melting peak for CaCl2x 6 H2O in alumina pores. The salt content is 35 % wt.
Samples requirements
The powder and liquid samples in volume not more than 40 mcL.
The crystalline and plate samples are not more than 6 mm in diameter
Leading scientists and their research interests
A.V. Gubar. Inorganic salts dispersed in porous matrix.
Detection of Free Radicals in Gas Phase
Objects of study
Heterogeneous reactions with hydrocarbon participation accompanied by the formation of free radicals and excited molecules in gas phase. The following reactions are of interest: complete and partial oxidation, hydrogenation and dehydrogenation, catalytic pyrolysis, alkylation and others.
Applications
Free radicals and excited molecules formed on a catalyst surface can initiate parallel gas-phase conversion of reagents. This process must be taken into account in the study of kinetics and reaction mechanism. Two methods have been developed for detection of radicals in gas phase, namely matrix isolation technique with EPR and direct mass-spectrometric detection.
Instrument facilities
- Matrix isolation technique. Radicals are detected with EPR. To accumulate the radicals a gas mixture after the reactor is frozen out in a collector cooled by liquid nitrogen mounted in an EPR resonator. Then the frozen condensate is irradiated with UV light and the radicals formed in the photolysis are studied. Products incondensable at 77 K are analyzed with MX-7301 mass-spectrometer. A flow reactor of ideal mixing with the pressure range from 1 to 20 Pa is applied. The sensitivity of the method is 108 radicals/cm3.
- Direct mass-spectrometric detection of free radicals. Mass-spectrometric set-up UR-1 with variable energy of ionizing electrons has been assembled. This setup allows to detect both radicals and stable products of a chemical reaction. A molecular beam of reaction products is directed into a mass-spectrometer with a sampling time less than 0.1ms. Simultaneous detection of 10 components is possible during a kinetic measurement. The flow reactor of ideal substitution with the pressure range from 100 to 105 Pa is applied. The method sensitivity by the methyl radicals is 1011 radicals/cmsup>3.
Leading scientists and their research interests
A.Yu. Gladky. Alcohol oxidation, intermediates in steam conversion of methane, propane pyrolysis.
Radioisotope Methods for the study of the mechanisms and reactions kinetics
Applications
- Isotope exchange method: study of the chemical conversion rate under the equilibrium conditions and assessment of the elementary reaction rate constants. It is possible to calculate various types of exchange (homoexchange, heteroexchange).
- Assessment of kinetic isotope effects: study of the limiting stage of the complicated chemical process and reaction mechanism.
- Kinetic isotope method: determination of the conversion sequence in the complicated process and measurement of the rate of reaction stages.
- Thermal desorption method with isotope application: study of the molecule fragmentation peculiarities at the interaction with the catalyst surface.
- Estimation of the diffusion coefficients with radioisotopes.
- Determination of the specific metal surface at a small value of the specific surface (from tens cm2).
Instrument facilities
SL-4221 scintillation beta-counter for estimation of the radioactivity of the sample containing tritium, C-14, S-35, P-32 and other beta-emitting isotopes, dissolved in the standard liquid scintillators. The sample volume: about 1 ml.
22 042 RFT gamma-spectrometer for recording of the emission and radioactivity spectra of the solid samples containing various gamma emmitters.
E50 Nuciear flow proportional counter for estimation of radioactivity of gas and vapor samples containing tritium or C-14. The counter is used simultaneously with gas-liquid chromatograph for continuos radioactivity analysis. The sample volume: about 1 ml of gaseous or 1 ml of liquid sample.
Leading scientists and their research interests
Dr. V.A. Rogov. Isotope methods in chemical kinetics.
Dr. G.D. Bukatov. Olefin polymerization.